As a leader in bringing research findings to clinical application, The Lundquist Institute conducts many clinical trials. Clinical trials are a method of objectively assessing whether novel therapies are safe and effective in treating disease.
Deciding whether to become a research participant is an important choice, a personal choice, and a voluntary one. It should also be an informed choice. You should have answers to all your questions before you choose.

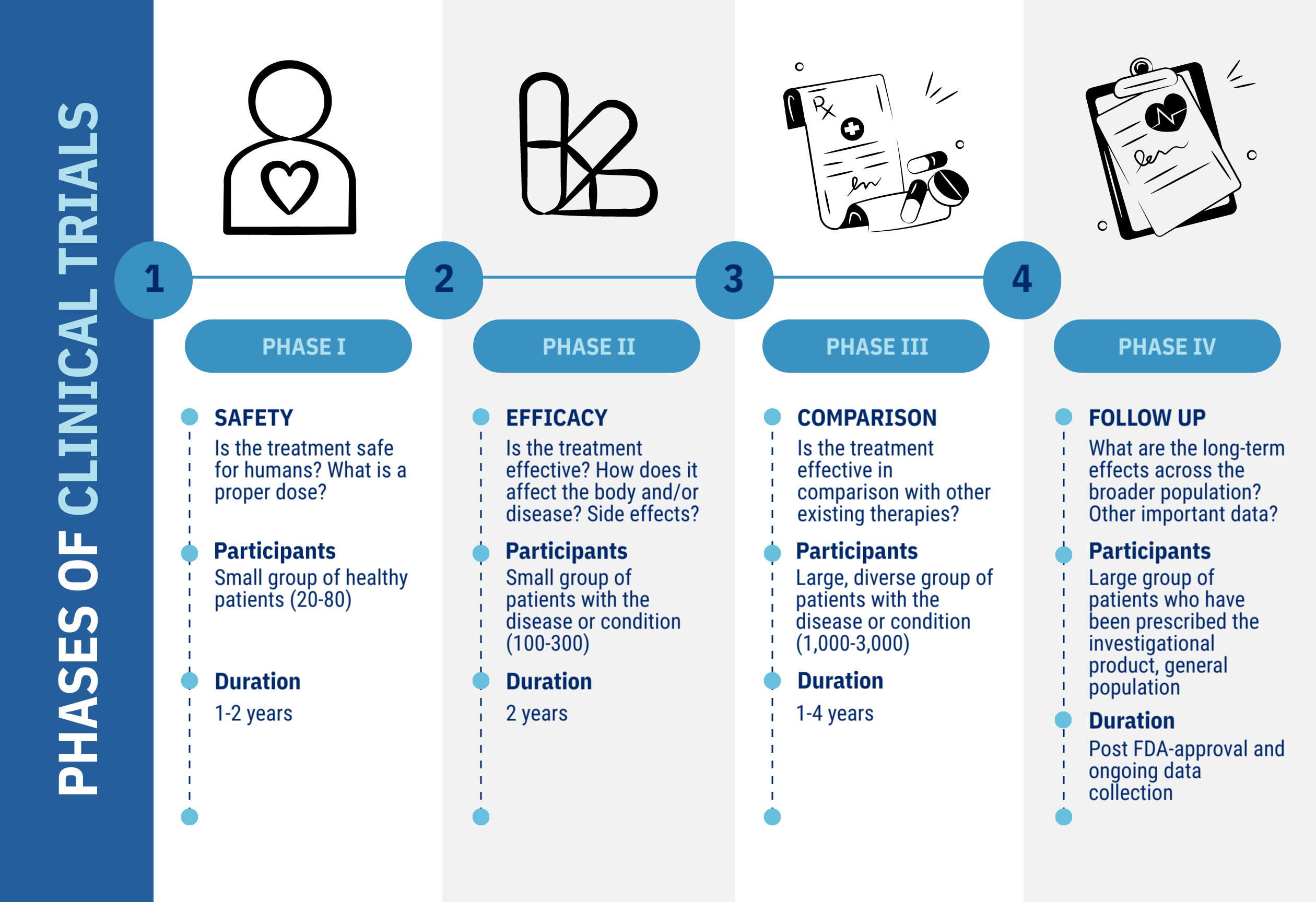
What is a clinical trial?
A clinical trial is a research study designed to test the safety and efficacy of a medical intervention, such as a new drug, medical device, or therapy. These studies help determine if a treatment works better than current options or has fewer side effects. Clinical trials can also explore ways to prevent diseases, diagnose conditions early, or improve the quality of life for those with chronic or life-threatening illnesses.
Before reaching human trials, potential treatments undergo rigorous safety and efficacy testing and must receive approval from the FDA in the United States. By participating, individuals contribute to scientific discovery and the development of better healthcare options for future generations.
Why does clinical trial participation matter?
Ensuring that clinical trial participants reflect the diversity of the local population is essential for developing treatments that work effectively across the communities they serve. Los Angeles County is one of the most diverse regions in the country, yet historically, clinical trials have underrepresented minority populations, leading to a lack of relevant data and outcomes for these groups. For example, despite the County’s large Hispanic, African American, and Asian populations, participation in clinical trials often falls short of mirroring this diversity, which impacts the safety and effectiveness of therapies developed for these patients.
This underrepresentation can result in treatments that are less effective or have unanticipated side effects for specific groups, contributing to persistent health disparities and fewer viable options for those who need them most. A lack of diversity also means researchers may miss critical variations in disease progression and drug response, ultimately affecting the quality of patient care. To address this, TLI is committed to expanding inclusive participation, ensuring that our clinical trials encompass a broad spectrum of racial, ethnic, and socioeconomic backgrounds.
What is it like to be a clinical trials participant?
For more information on participating in a clinical trial, please visit our For Research Participants page.
Want to get involved in active trials at TLI?
For information on privately and publicly funded clinical trials at The Lundquist Institute and worldwide, please visit www.clinicaltrials.gov. Searching by “disease AND Lundquist” will identify trials that The Lundquist Institute may be conducting for a particular condition.
TLI conducts disease-specific clinical trials and studies involving healthy participants, each playing a vital role in advancing biomedical research. We welcome your involvement if you’re interested in a particular study, want to explore trials currently recruiting, or represent a company looking to collaborate on clinical research.
Please get in touch with our Clinical Trials and Industry Agreements team at 323-457-1440 or [email protected] to learn more about how to take the next step.