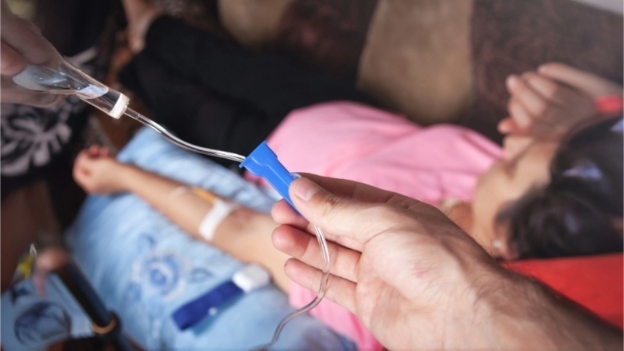
Staphylococcus aureus is not just painful and uncomfortable, but deadly if untreated. Commonly referred to as a “staph infection,” the severity of S. aureus is rooted in its ability to enter the bloodstream and spread throughout the body, leading to life-threatening effects like sepsis, pneumonia, endocarditis, or osteomyelitis. These infections can affect healthy persons or strike vulnerable persons in the hospital who have weakened immune systems. Standard treatment relies on antibiotics administered intravenously (IV) over several weeks. Many patients sent home after being hospitalized for staph infections require home IV antibiotics, which typically require multiple daily infusions through a special IV and increase the risk for blood clots and additional infections.
However, Loren Miller, MD, MPH, investigator at The Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, and other researchers have reason to believe that dalbavancin, a novel antibiotic treatment requiring only two doses over four to eight weeks, could be a viable alternative.
In a paper recently published in JAMA, Dr. Miller and his colleagues found that dalbavancin works as well as standard treatments for S. aureus. This means that for this common infection, patients can be freed of home IVs and all the inherent inconvenience and risks of a long-term IV. In this study, 200 adults were recruited across 23 United States and Canada sites–one of which was The Lundquist Institute in Torrance, California.
To understand if dalbavancin is a superior treatment option for complicated S. aureus bacteremia compared with standard care, the team randomly assigned participants: 100 participants received standard treatment which entailed IV-administered antibiotics for four to eight weeks on a daily basis, and 100 participants who received two IV-administered doses of dalbavancin, one at the hospital when discharged and another one week later.
Data from day 70 of the trial showed that both groups reveal similar quality-of-life measurements, but participants in the dalbavancin group yielded a similar probability of having a more desirable outcome. While dalbavancin was not proven to be significantly worse nor better than standard treatment, findings suggest that this drug can be added to the armamentarium of treatments for this common and very serious infection.
Dalbavancin means that patients can be free from IVs in their arm for weeks to over a month. It also means they don’t need to infuse themselves at home, visit IV infusions centers, or have additional inconveniences of long-term IV therapy.
The study was sponsored by the National Institutes of Health (NIH) and the makers of dalbavancin were not involved in study conduct. To read the paper, “Dalbavancin for Treatment of Staphylococcus aureus Bacteremia,” published in Jama Network, visit https://jamanetwork.com/journals/jama/article-abstract/2837604.