Lundquist Institute's Dr. Eric Daar Lead Author in Paper in the Annals of Internal Medicine
The study showed that antibody combination provides strong protection against severe COVID-19 in large international trial. The trial enrolled non-hospitalized patients with early, mild to moderate COVID-19 who were considered at high risk of progression to severe COVID-19. The participating medical centers were in Argentina, Brazil, Mexico, the Philippines, South Africa, and the United States.
The study showed that antibody combination provides strong protection against severe COVID-19 in large international trial. A treatment combining two antibodies against the coronavirus SARS-CoV-2 strongly protected high-risk people with early COVID-19 symptoms from hospitalization and death in an international Phase 2/3 clinical trial. The trial enrolled more than 800 non-hospitalized patients with COVID-19 at high-risk of progression of the disease in the United States and five other countries.
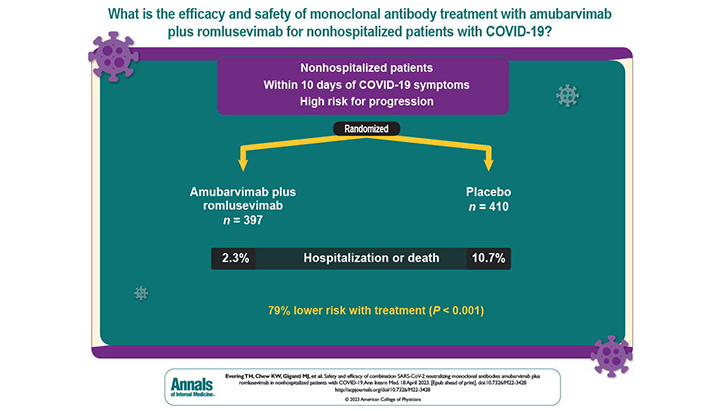
Those who were randomly assigned to be treated with the combination of the two antibodies, amubarvimab and romlusevimab, had only a 2.3 percent rate of progression to hospitalization and/or death, compared to 10.7 percent in the placebo group, a highly significant difference. The treatment also appeared safe.
The study, which is part of the ACTIV-2 Study of Outpatient Monoclonal Antibodies and Other Therapies was also co-led by researchers at the University of California-San Diego, The Geffen School of Medicine at UCLA, and The Lundquist Institute at Harbor-UCLA Medical Center. ACTIV-2 is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH) and is part of NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) public-private partnership.
The trial enrolled non-hospitalized patients with early, mild to moderate COVID-19 who were considered at high risk of progression to severe COVID-19. The participating medical centers were in Argentina, Brazil, Mexico, the Philippines, South Africa, and the United States. Enrollment took place during January to July of 2021, and the final analysis covered 807 patients, the vast majority of whom were from the United States, South Africa, or Argentina.
Although the treatment and placebo groups were roughly equal in number (397 and 410), 44 of the 53 total hospitalizations and/or deaths occurred in the placebo group, compared with only 9 in the treatment group—a 79 percent reduction in risk for the latter. The analysis also found that the antibody treatment worked about as well for patients enrolled 6 to 10 days after symptom onset as it did for patients enrolled earlier. It also appeared to be effective against the delta variant of SARS-CoV-2, which emerged and spread worldwide during the study. Learn more here: https://www.acpjournals.org/doi/10.7326/M22-3428
