The Lundquist Institute at Harbor-UCLA Medical Center Administers First Phase 3 AstraZeneca Trial Injection
First participants received the shot at The Lundquist Institute on Friday, September 4; more injections scheduled next week
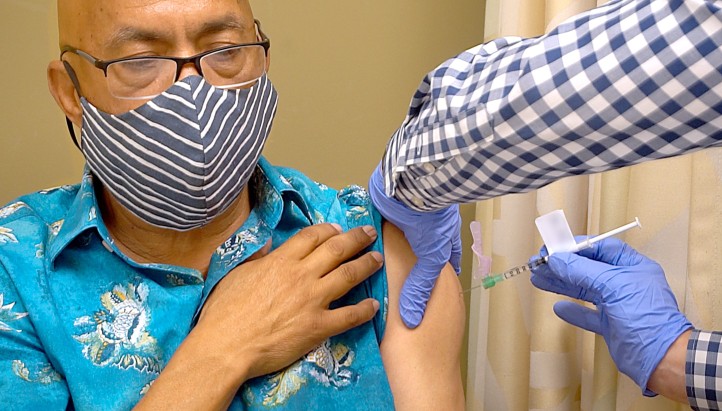
LOS ANGELES – The Lundquist Institute at Harbor-UCLA Medical Center officially began phase 3 trials of the Oxford University – AstraZeneca COVID-19 vaccine trial on Friday, September 4.
The vaccine is called AZD1222 and has already gone through early trials. This trial is designed to determine whether the vaccine can prevent symptomatic COVID-19 after two doses. The study aims to enroll 30,000 volunteers across the United States. It is important to note that this vaccine does not have a live virus and cannot give a person COVID-19. If the results from the trial are positive, the vaccine could be made available to the public to prevent disease.
On Friday, 5 participants received an injection with additional participants scheduled for after the Labor Day Holiday.
To be eligible, a volunteer cannot have tested positive for COVID-19. Volunteers are assigned at random to receive a placebo (saline injection) or the investigational vaccine. The trial is blinded, meaning the participants and the investigators will not know who receives the vaccine. Of the volunteers enrolled, two-thirds will get the study vaccine and one-third will get a placebo or harmless injection. This allows researchers to compare outcomes in the vaccine group versus the placebo group. In the two years that follow, researchers will monitor all study volunteers for the development of symptomatic COVID-19.
“We are excited to have this very important study underway. Identifying an effective vaccine won’t be possible without the support of the community. We’re incredibly pleased with the number of individuals who have already volunteered to take part but we still need many more volunteers,” said Dr. Eric Daar, Lead Researcher for The Lundquist Institute on this trial, Chief of HIV Medicine at the Harbor-UCLA Medical Center and professor at UCLA’s Geffen School of Medicine. “Help Stop COVID LA is an opportunity for the Los Angles community to come together and make a meaningful impact towards ending this pandemic.”
The first participant to receive an injection in Los Angeles was Jorge Vega, a 62-year-old, LatinX male who works as a waiter in Los Angeles.
“This is important because it’s my way to help my family and my community. I know too many co-workers who have died from COVID,” said Jorge Vega, the first person in LA to receive a trial injection. “To my community, I say don't be afraid. This is for the well-being of humanity.”
The Lundquist Institute and UCLA Medical Center in Westwood are two sites that are both part of the COVID-19 Prevention Network (CoVPN) participating in this phase 3 trial. The clinical research network, supported by the National Institute of Allergy and Infectious Diseases (NIAID), part of NIH, aims to enroll thousands of volunteers in large-scale clinical trials testing a variety of investigational vaccines and monoclonal antibodies intended to protect people from COVID-19.
The Lundquist Institute and UCLA Westwood are continuing to recruit volunteers who reside in Los Angeles County, primarily including members of communities of color that have been disproportionately affected by COVID-19 and individuals at high-risk for severe disease. This includes:
- Persons over 60 years of age;
- Persons with preexisting medical conditions including high blood pressure, diabetes and obesity.
To find out more and sign up to volunteer please visit www.helpstopcovid.la.
