Taking aim at one of the nation’s most common - but understudied - diseases, a team of local investigators have launched a groundbreaking study to determine the effectiveness of treatments for antibiotic-resistant skin infections.
The investigators from LA BioMed, one of the nation’s leading independent nonprofit research institutes, have been awarded a $5.3 million grant from the National Institutes of Health for their study, Short and Long Term Outcomes of Doxycycline Versus Trimethoprim-Sulfamethoxazole for Skin and Soft Tissue Infections Treatment.
Mucormycosis is a drug-resistant fungal infection that attacks patients with weakened immune systems and those who have suffered significant trauma. The infection can be easily missed by physicians because it is so rare and reliable diagnostic assay is lacking. Even when correctly diagnosed, it is often far too late and after the infection has spreads rapidly to vital organs, making most therapies ineffective. The mortality rate for those infected with mucormycosis is around 50%, according to the U.S. centers for Disease Control and Prevention.
LA BioMed President and Chief Executive Officer David Meyer, Ph.D., is set to elaborate on the future of the local bioscience industry during a panel discussion later today at the Los Angeles County 2018 Bioscience Summit. Meyer will also moderate a panel discussion about incubation and capital for start-ups.
LA BioMed, an independent biomedical research organization, hosted its fifth annual Innovation Showcase on September 13th in Manhattan Beach. The event featured some of biotechnology’s most promising start-ups and spotlighted 32 promising new bioscience companies.
LA BioMed invites media to their fifth annual Innovation Showcase on September 13th at westdrift in Manhattan Beach, CA. The Showcase will highlight some of biotechnology’s most promising start-ups and venture capital leaders.
Biotech start-up entrants from the growing incubation hub of LA will showcase innovation in next generation medical therapies, devices and diagnostics from some of the worlds’ leading scientists. Highlights from the 32 featured companies include:
LA BioMed, an independent biomedical research organization, announced it will host its fifth annual Innovation Showcase on September 13th at westdrift Manhattan Beach. The Showcase highlights biotechnology’s most promising start-ups and venture capital leaders.

LA BioMed announced that it has closed on the sale of its $49,835,000 Series 2018 Tax-Exempt Revenue Bonds issued by the California Health Facilities Financing Authority (CHFFA). The proceeds of the revenue bond sale, which was led by Wells Fargo Securities, will be used to finance various capital improvements on the LA BioMed campus, including constructing and equip
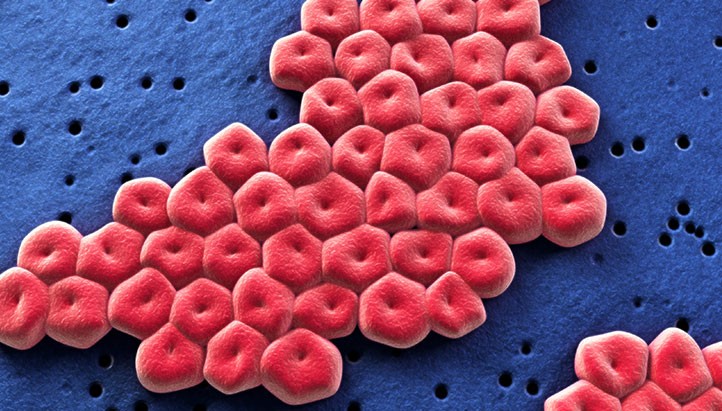 A groundbreaking study by LA BioMed researchers shows promising results for a potential vaccine for deadly drug-resistant bacteria also known as superbugs.
A groundbreaking study by LA BioMed researchers shows promising results for a potential vaccine for deadly drug-resistant bacteria also known as superbugs.
LA BioMed announced the launch of two new male contraceptive studies. The preliminary study of the pill showed that the oral contraceptive appears to be safe and effective when taken daily. Current methods of male contraception include vasectomy, condoms, withdrawal, and fertility awareness.



